The Ri has been the place where science lives for over 200 years, home to pioneering chemists like Humphry Davy and Lord Rayleigh. A whopping 10 chemical elements are associated with our history - either discovered or isolated within our walls in Mayfair, or by people who were working at the Ri.
As the 2022 CHRISTMAS LECTURES with Sue Black shine a light on forensic science, we take a look at how some these chemical elements are used in the field, from identifying bodies to catching criminals.
Potassium
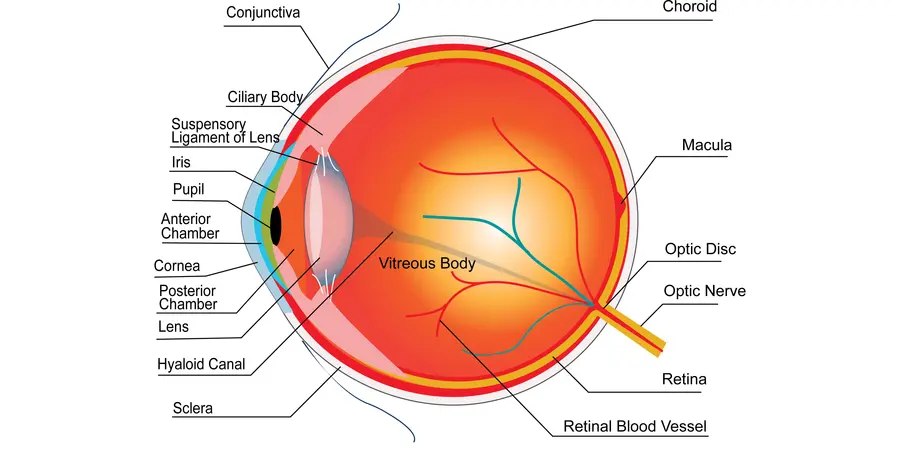
One way to determine the time of death of a body is by measuring the potassium ions in a part of the eye called the vitreous body (the gel-like area between the lens and the retina).
The level of vitreous potassium generally increases after death, as potassium from surrounding areas moves into the vitreous body. However, the rate of increase depends on other factors such as age, temperature and alcohol levels at the time of death.
The concentrations of sodium, calcium, and magnesium, all also discovered at the Ri, have also been found to estimate time of death. With research for these elements is much less consistent, vitreous potassium remains the most established method.
Potassium was first isolated by Humphry Davy in 1807 using electrolysis, a technique using electric current to drive chemical reactions. The name derives from the word ‘potash’, an early process of extracting potassium salts.
Sodium and barium
When a gun is fired, it produces gunshot residue (GSR) in the form of a powder deposited in the close vicinity of the shooting and would be found on the hands and clothes of the person firing, or the clothes of the target. Three Ri chemical elements are linked to this.
Lead, antimony and barium are indicators of GSR, as it is unlikely to find the combination of these three elements in any other substance. They make up particles of the primer found in GSR, which is responsible for producing the flame that ignites the detonator in the gun.
Sodium can be used to detect the presence of lead in GSR, through the sodium rhodizonate test.
If lead is present, a red/purple colour is produced by the test, alerting to the presence of GSR. However, conducting this analysis at the crime scene is risky as cross-contamination can occur, removing any presence of GSR, therefore it is crucial to test as soon as possible after the crime has been committed, with as little crime scene intervention as possible. Tests such as the sodium rhodizonate test that involve a colour change are not as heavily relied upon by forensic scientists, as more advanced methods have been developed to ensure confident results.
Sodium and barium were both first isolated by Humphry Davy between 1807 and 1808, once again using electrolysis.

Strontium
Strontium naturally builds up in the bones and teeth over the course of your life. As it is found in the soil and water, it's integrated into our food chain, and subsequently into our teeth. However, not everyone's strontium composition is the same, and it varies depending on where one grows up.
Different forms of strontium, known as isotopes, are found in varying ratios around the world. Therefore, the ratio of isotopes that exists where you grow up integrates itself into your teeth and can serve as a sort of geological fingerprint. Teeth develop at different ages, so forensic scientists can use strontium to trace different stages of life to where someone was living at the time. Comparing this to the place of death could provide information about a victim's lifestyle or the circumstances of their death.
This is becoming increasingly challenging as people's diets become more 'international' due to importing food, but one thing remains the same and is locally sourced – drinking water.
Strontium was also isolated by Humphry Davy in 1808 (what a busy year he had!) The element is named after the Scottish village of Strontian, where it was discovered in lead mines.

Iodine
Iodine is used in the development of latent fingerprints—prints not visible to the naked eye, produced by oil and sweat transferred from the body onto surfaces.
A quick and cost-efficient technique to reveal latent prints both in the lab or at the crime scene is iodine fuming, which turns the prints a brownish colour. However, this visualisation is temporary, and scientists need to be ready to either photograph the fingerprints before they disappear or fix them with a chemical called benzoflavone.
Iodine was originally discovered by Bernard Courtois in 1811, however, he lacked the resources to isolate it. At the time, scientists thought it was either a compound of oxygen or an element. Humphry Davy was the one to find the elemental nature of iodine later that same year.

Argon
Argon is used in forensics in the form of argon ion lasers. Ion lasers are a type of gas laser, which function by passing an electric current through a gas, producing the laser beam. Using ionized gases means that the gas used in the laser has an electrical charge.
Argon ion lasers can function at a wide range of wavelengths. This allows lasers to photograph latent fingerprints in detail, especially those containing blood. These lasers are extremely useful in capturing images of shoeprints at crime scenes, particularly on surfaces such as wood, plastic and vinyl. The wavelength at which the laser functions also allows for the visualization of blood in shoeprints.
Argon ion lasers are used when all usual methods of detecting erased writing have failed – this includes UV light, infrared and infrared luminescence.
Argon was discovered by Lord Rayleigh and William Ramsay in 1894. It was the first of the noble gases to be discovered, and it gets its name from the Greek for 'lazy, inactive, due to its very low chemical reactivity.
Uncover the secrets of forensic science
For the 2022 CHRISTMAS LECTURES, forensic anthropologist Sue Black will take us along as she illuminates crime scene investigation and opens the doors of leading-edge forensic labs, revealing the most interesting and fascinating aspects of forensic science. Catch the lectures on BBC Four between Christmas and New Year.

About the author
Lia Hale is an MSc Science Communication graduate from Imperial College London, and works in the Ri digital team following a summer placement as a Digital Media Intern.
