Lecture 3 – Semiconductors, superconductors and catalysts
From the programme:
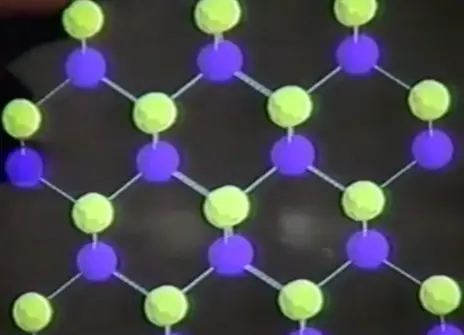
A metal such as copper, in marked contrast to an insulator such as mica, conducts electricity freely, and its resistance drops with decreasing temperature. But some crystals, as Faraday discovered in 1839 have intermediate powers of conduction, and their resistance decreases with increasing temperature. These are the so-called semiconductors, nowadays the key components of sophisticated electronic circuits. Some other crystals exhibit the remarkable phenomenon, currently of exceptional and worldwide interest, of losing all their resistance as the temperature drops. These are the superconductors. We shall demonstrate how they could revolutionize fields as distinct as medical diagnostics and transportation.
Semiconductors often respond ultra sensitively to light, a fact that is put to good use in the construction of both radiation detectors and solar cells and in the production of infrared cameras. Semiconductors, when appropriately designed in both the chemical and engineering sense, can also function as efficient lasers.
Catalysts are capable of effecting highly efficient chemical conversions and, depending upon the reaction in question, a crystalline catalyst may be a metal, a semiconductor, or an insulator. We shall illustrate the remarkable performance of two particular crystalline catalysts: platinum and a man-made version of minerals known as zeolites.
About the 1987 CHRISTMAS LECTURES
From the 1987 lecture programme:
"Crystals first appeared on earth many millions of years before the emergence of life. The first laser, however, based on a man-made crystal of ruby, was fashioned less than thirty years ago. Nowadays, crystals and lasers play crucial roles in the design of new materials, in the expanding world of communications, in medicine and environmental science and in the quest for better, cleaner forms of energy. They also function as the picot for a wide range of fundamental scientific studies."
In this series of six CHRISTMAS LECTURES, Professors John Meurig Thomas and David Phillips explore the science and applications of crystals and lasers, reviving some of the key experiments from the history and evolution of physical sciences and exploring what the future might bring.